 首页-
Products -
Others -
Other Targets -
4-fluoro-N-(3-fluoro-4-methylphenyl)-3-{[(4-methoxyphenyl)methyl]sulfamoyl}benzamide
首页-
Products -
Others -
Other Targets -
4-fluoro-N-(3-fluoro-4-methylphenyl)-3-{[(4-methoxyphenyl)methyl]sulfamoyl}benzamide
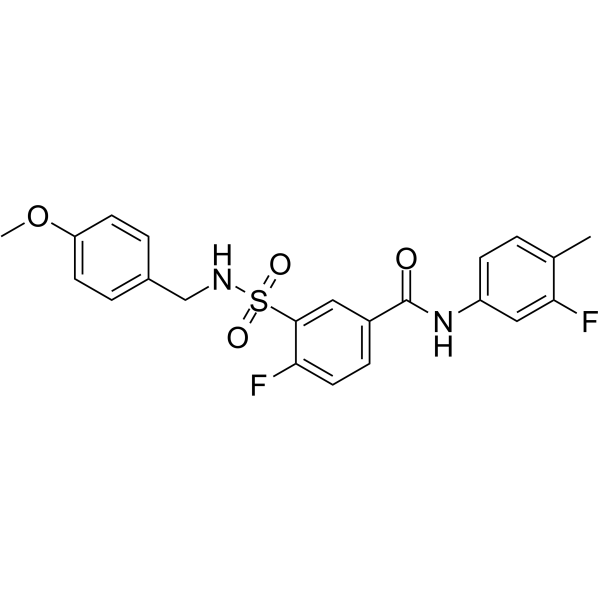
4-fluoro-N-(3-fluoro-4-methylphenyl)-3-{[(4-methoxyphenyl)methyl]sulfamoyl}benzamide
CAS No. 422546-87-0
4-fluoro-N-(3-fluoro-4-methylphenyl)-3-{[(4-methoxyphenyl)methyl]sulfamoyl}benzamide ( —— )
产品货号. M28461 CAS No. 422546-87-0
4-氟-N-(3-氟-4-甲基苯基)-3-{[(4-甲氧基苯基)甲基]氨磺酰基}苯甲酰胺是一种用作分子结构单元的化合物。
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 5MG | ¥413 | 有现货 |


|
| 10MG | ¥583 | 有现货 |


|
| 25MG | ¥956 | 有现货 |


|
| 50MG | ¥1409 | 有现货 |


|
| 100MG | ¥2155 | 有现货 |


|
| 200MG | 获取报价 | 有现货 |


|
| 500MG | 获取报价 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称4-fluoro-N-(3-fluoro-4-methylphenyl)-3-{[(4-methoxyphenyl)methyl]sulfamoyl}benzamide
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述4-氟-N-(3-氟-4-甲基苯基)-3-{[(4-甲氧基苯基)甲基]氨磺酰基}苯甲酰胺是一种用作分子结构单元的化合物。
-
产品描述4-fluoro-N-(3-fluoro-4-methylphenyl)-3-{[(4-methoxyphenyl)methyl]sulfamoyl}benzamide is a compound used as a molecular building block.
-
体外实验——
-
体内实验——
-
同义词——
-
通路Others
-
靶点Other Targets
-
受体I2-imidazoline receptor
-
研究领域——
-
适应症——
化学信息
-
CAS Number422546-87-0
-
分子量446.467
-
分子式C22H20F2N2O4S
-
纯度>98% (HPLC)
-
溶解度In Vitro:?DMSO : 125 mg/mL (279.97 mM)
-
SMILESCOc1ccc(CNS(=O)(=O)c2cc(ccc2F)C(=O)Nc2ccc(C)c(F)c2)cc1
-
化学全称——
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
产品手册




关联产品
-
Cyclo(Phe-Hpro)
Cyclo(Phe-Hpro) is a natural product for research related to life sciences.
-
3-O-[5-O-feruloyl-be...
3-O-[5'''-O-feruloyl-beta-D-apiofuranosyl(1'''->2'')-beta-D-glucopyranosyl] rhamnocitrin is a natural product for research related to life sciences.
-
DM1-MCC
DM1-MCC 是一种带有 MCC 连接子的抗癌药物 DM1。



 021-51111890
021-51111890 购物车()
购物车()
 sales@molnova.cn
sales@molnova.cn






